Background: Allogeneic hematopoietic cell transplantation (Allo-HCT) is a highly effective method of achieving prolonged disease control for many types of blood cancers. However, historically, older adults were not considered eligible candidates for Allo-HCT due to concerns about increased toxicity and mortality. This is a significant issue, as the proportion of cancer patients with comorbidities, disability, and geriatric syndromes increases by 3-5% for every 5-year increase in age beyond 65 years. This systematic review aims to address this gap in literature by analyzing survival prognosis in patients aged 70 years and above who have undergone Allo-HCT.
Methods: As per the preferred reporting items for systemic reviews and meta-analysis (PRISMA) guidelines, a comprehensive literature search was performed on 3 databases (PubMed, Cochrane Register of Controlled Trials and Clinicaltrials.gov) using MeSH terms and keywords for “Hematopoietic Stem Cell Transplantation” AND “Outcome Assessment” AND “>70 years old” from the date of inception to Feb 13, 2023. Our research produced 101 articles. After excluding irrelevant and review articles during primary and secondary screening, six original studies reporting outcomes of Allo-HCT in patients over 70 years were included. The methodological quality of the included studies was evaluated using NIH quality assessment tool. Inter-study variance was calculated using the Der Simonian-Laird Estimator. Proportions along with 95% confidence Interval (CI) were extracted to compute pooled analysis using the ‘meta’ package by Schwarzer et al. in the R programming language (version 4.16-2).
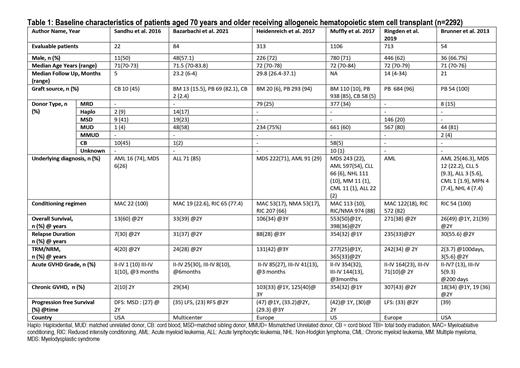
Results: A total of 2292 patients aged 70 years or more were included for this systematic review and meta-analysis. (Table 1) The median age was 71.75 (70-84) years and 67.5% (n=1547) patients were male. Median follow-up time was 22.1 (5-37.1) months. The underlying diagnosis was acute myeloid leukemia (AML) 63% (n=1445), myelodysplastic syndrome (MDS) 20.9% (n=480), non-Hodgkin lymphoma (NHL) 5% (n=115), acute lymphocytic leukemia (ALL) 4.2% (n=96), and chronic lymphocytic leukemia (CLL) 3.1 % (n=71). The source of stem cells was peripheral blood, bone marrow, and cord blood in 90.2% (n=2068), 6.2% (n=142), and 3.1% (n=70) of the patients, respectively. Myeloablative conditioning (MAC) was used in 17.8% of patients, reduced-intensity conditioning (RIC) was used in 77.4% of patients, and a non-myeloablative conditioning regimen (NMA) was used in 4.6% of patients. The Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) of the included patients was 0 (25.7%), 1-2 (30.1%), and 3 or more (44.2%). The pooled overall survival (OS) at a median of 2 years (1-3) was 41.8% (95% CI 0.34-0.49, I2=88%, p<0.01, n=2292). The pooled progression-free survival (PFS) was 36.4% (95% CI 0.31-0.43, I2=75%, p<0.01, n=1979) at a median of 2 (1-2) years while the pooled incidence of relapse and non-relapse mortality (NRM) was 33.7% (95% CI 0.29-0.38, I2=69%, p<0.01, n=2292) and 28.7% (95% CI 0.22-0.36, I2=88%, p<0.01, n=2292), respectively. The pooled incidence of acute graft versus host disease (GvHD) grade II-IV at a median of 6 months (3-24) and grade III-IV at median of 4.5 months (3-24) was 25.8% (95% CI 0.20-0.31, I2=84%, p<0.01, n=2270) and 11.1% (95% CI 0.10-0.13, I2=2%, p=0.39, n=1979), respectively, while the pooled incidence of chronic GvHD at a median of 1.5 years (1-2) was 33.6% (95% CI 0.28-0.40, I2=84%, p<0.01, n=2292).
Conclusion: Our meta-analysis shows that allo-HCT offers promising outcomes for elderly patients aged 70 or greater, and should be considered more frequently for those with transplant eligible disease. It also shows that age can be a restrictive factor due to co-existing comorbidities and poor performance status. Several other factors such as the availability of matched donors, conditioning regimen can play a role in the final outcome of disease progression and associated adverse effects such as GVHD.
Disclosures
Jaglal:Agios: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; SOBI: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal